general tests ptce practice test
Pharmacy Technician Certification (CPhT) Exam
Question 1
The ingredients of 1 kg of a bulk laxative are:
Psyllium:500 g
Dextrose:497.5 g
Citric acid:1 g
Sodium bicarbonate:1 g
Flavoring:0.5 g
What is the percentage ofpsylliumin the final preparation?
- A. 2.5%
- B. 5%
- C. 25%
- D. 50%
Answer:
D
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
To calculate the percentage ofpsylliumin the final preparation:
Formula: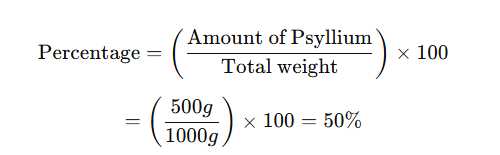
A math equations with numbers and symbols Description automatically generated with medium
confidence
Sincepsylliummakes up500 gout of1000 g (1 kg)of the bulk laxative, it accounts for50%of the total
formulation.
Reference:
USP <795>Compounding Standards
PTCE ExamPharmaceutical Calculations
Question 2
Levetiracetam is a(n):
- A. Antibiotic
- B. Antihyperglycemic
- C. Anticonvulsant
- D. Antihypertensive
Answer:
C
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Levetiracetam (Keppra)is classified as ananticonvulsantused to treatseizures (epilepsy).It works by
stabilizing electrical activity in the brain.
✅
Explanation of Answer Choices:
C. Anticonvulsant→Correct. Levetiracetam is indicated forpartial-
❌
onset, myoclonic, and tonic-clonic seizures.
A. Antibiotic→ Incorrect. Levetiracetam does not treat
❌
bacterial infections.
B. Antihyperglycemic→ Incorrect. Antihyperglycemics lowerblood sugar(e.g.,
❌
metformin, glipizide).
D. Antihypertensive→ Incorrect. Antihypertensives lowerblood
pressure(e.g., amlodipine, lisinopril).
Reference:
PTCB Exam: Pharmacology for Technicians
FDA Approved Drug Database (Levetiracetam)
Question 3
Behind-the-counter decongestant products containingpseudoephedrinemust be used with caution in
patients with:
- A. Asthma
- B. Hypertension
- C. Hypokalemia
- D. Eczema
Answer:
B
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Pseudoephedrine(Sudafed) is asympathomimetic decongestantthat causesvasoconstrictionand
canincrease blood pressure.
✅
Explanation of Answer Choices:
B. Hypertension→Correct.Pseudoephedrinecan cause arise in
❌
blood pressure, so it should be used cautiously in patients withhypertension.
A. Asthma→
Incorrect.Pseudoephedrine does not directly worsen asthma, but it may cause mild
❌
bronchodilation.
C. Hypokalemia→ Incorrect.Pseudoephedrine does not affect potassium
❌
levels.
D. Eczema→ Incorrect. Eczema is unrelated topseudoephedrine use.
Reference:
Combat Methamphetamine Epidemic Act (CMEA) Regulations
American Heart Association (AHA) Guidelines on Hypertension
Question 4
Due to an increased risk of hepatotoxicity, patients on acetaminophen should use caution when
consuming:
- A. Citrus fruits
- B. Leafy greens
- C. Dairy products
- D. Alcoholic beverages
Answer:
D
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Acetaminophen (Tylenol)is metabolized by theliver.Excessive doses or concurrentalcohol
useincreases the risk ofliver damage (hepatotoxicity).
✅
Explanation of Answer Choices:
D. Alcoholic beverages→Correct. Alcohol and acetaminophen
❌
together can causesevere liver damage.
A. Citrus fruits→ Incorrect. Citrus does not interfere
❌
withacetaminophen metabolism.
B. Leafy greens→ Incorrect. Leafy greens affectwarfarin,
❌
notacetaminophen.
C. Dairy products→ Incorrect. Dairy does not interact withacetaminophen.
Reference:
FDA Acetaminophen Warnings
PTCB Medication Safety Guidelines
Question 5
A prescription reads:
Famotidine 40 mg/5 mL
Quantity: 50 mL
Sig: 0.4 mL PO t.i.d.
What amount of medication, in mg, is given each day?
- A. 1.2 mg
- B. 3.2 mg
- C. 6.4 mg
- D. 9.6 mg
Answer:
C
Explanation:
Comprehensive and Detailed Step-by-Step Explanation: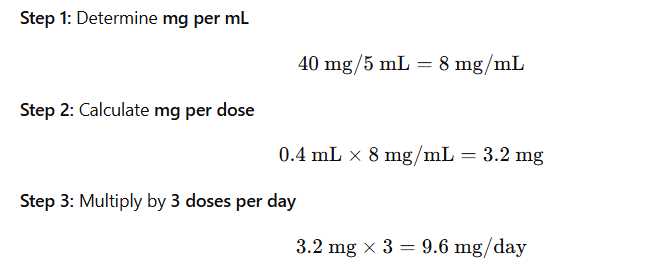
A table with text and numbers Description automatically generated with medium confidence
Reference:
PTCB Exam: Pharmaceutical Calculations
USP <795> Dosing Conversions
Question 6
According toUSP Chapter 800, a reusable counting tray used to count outcyclophosphamidecapsules
must be:
- A. Activated before each use.
- B. Disposed of after each use.
- C. Decontaminated after each use.
- D. Sterilized before each use.
Answer:
C
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Cyclophosphamideis ahazardous drugclassified byNIOSH (National Institute for Occupational Safety
and Health).USP Chapter 800mandates thatall surfaces exposed to hazardous drugs (HDs), including
counting trays, must be decontaminated after each useto prevent cross-contamination.
✅
Explanation of Answer Choices:
C. Decontaminated after each use→Correct.Hazardous drug
❌
residues must be removed after each use to prevent contamination.
A. Activated before each
❌
use→ Incorrect. There is no"activation"process for hazardous drug counting trays.
B. Disposed of
❌
after each use→ Incorrect.Counting trays are reusable but must be properly cleaned.
D. Sterilized
before each use→ Incorrect.Sterilization applies to sterile compounding, not hazardous drug
handling.
Reference:
USP <800>: Handling Hazardous Drugs in Healthcare Settings
NIOSH List of Hazardous Drugs (Cyclophosphamide)
Question 7
Adispensing erroris defined as a discrepancy between a prescription and the medication that is:
- A. Requested by the patient.
- B. On the plan formulary.
- C. Supplied by the wholesaler.
- D. Received by the patient.
Answer:
D
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Adispensing erroroccurs when a pharmacy provides amedication that differs from the prescribed
order.This may involvewrong drug, dose, form, or incorrect labeling.
✅
Explanation of Answer Choices:
D. Received by the patient→Correct.Adispensing error is an error
❌
in what the patient actually receives, not what they request.
A. Requested by the patient→
❌
Incorrect.A patient request does not determine dispensing errors.
B. On the plan formulary→
❌
Incorrect.Formulary status affects insurance, not dispensing accuracy.
C. Supplied by the
wholesaler→ Incorrect.Errors in supply do not qualify as dispensing errors.
Reference:
ISMP Guidelines on Medication Errors
PTCB Medication Safety Guidelines
Question 8
Medications’lot numbersare assigned by the:
- A. Food and Drug Administration (FDA).
- B. Drug Enforcement Administration (DEA).
- C. Wholesaler.
- D. Manufacturer.
Answer:
D
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Alot number (or batch number)is aunique identifierassigned to a specific batch of medication by
themanufacturer. It helps trackrecalls, expiration dates, and quality control.
✅
Explanation of Answer Choices:
D. Manufacturer→Correct.Manufacturers assignlot numbersfor
❌
tracking and recall purposes.
A. FDA→ Incorrect. TheFDA does not assign lot numbersbut
❌
regulatesdrug safety and labeling.
B. DEA→ Incorrect. TheDEA controls controlled substancesbut
❌
does not issuelot numbers.
C. Wholesaler→ Incorrect.Wholesalers distribute drugs but do not
assign lot numbers.
Reference:
FDA Drug Product Recall Guidelines
PTCB Inventory Management Section
Question 9
According tofederal law, a prescription for which of the following medicationsmay be transferredto
another pharmacy to be refilled?
- A. Alprazolam
- B. Methylphenidate
- C. Fentanyl
- D. Hydromorphone
Answer:
A
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Underfederal law (21 CFR §1306.25),Schedule III-V controlled substances (e.g., Alprazolam)can be
transferred ONCEfor refill purposes between licensed pharmacieswith a shared database.Schedule II
drugs (e.g., Methylphenidate, Fentanyl, Hydromorphone) CANNOT be transferred.
✅
Explanation of Answer Choices:
A. Alprazolam (C-IV)→Correct.Schedule III-V drugs can be
❌
transferred ONCEfor refill purposes.
B. Methylphenidate (C-II)→ Incorrect.Schedule II drugs
❌
cannot be transferred.
C. Fentanyl (C-II)→ Incorrect.C-II drugs are non-transferablebetween
❌
pharmacies.
D. Hydromorphone (C-II)→ Incorrect.C-II drugs cannot be transferred.
Reference:
DEA Controlled Substance Act (CSA)
21 CFR §1306.25 – Prescription Transfers
Question 10
Which of the following products is acorticosteroid?
- A. Progesterone
- B. Fluocinolone
- C. Methyltestosterone
- D. Spironolactone
Answer:
B
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Corticosteroidsare a class of medications used toreduce inflammation.Fluocinoloneis atopical
corticosteroidused foreczema, psoriasis, and allergic reactions.
✅
Explanation of Answer Choices:
B. Fluocinolone→Correct.Fluocinolone is a corticosteroidused in
❌
❌
skin conditions.
A. Progesterone→ Incorrect.Progesterone is a hormone, not a corticosteroid.
C.
❌
Methyltestosterone→ Incorrect.Methyltestosterone is an androgen (male hormone).
D.
Spironolactone→ Incorrect.Spironolactone is a potassium-sparing diuretic, not a steroid.
Reference:
FDA Drug Classification Database
USP Drug Information Handbook
Question 11
The pharmacy must have the results of a currentANC (Absolute Neutrophil Count) and WBC (White
Blood Cell) blood testprior to dispensing which medication?
- A. Aripiprazole
- B. Fluoxetine
- C. Amitriptyline
- D. Clozapine
Answer:
D
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Clozapine (Clozaril)is anatypical antipsychoticused to treatschizophrenia. However, it has a severe
side effect calledagranulocytosis, which causesa dangerous drop in white blood cells (WBCs) and
neutrophils (ANCs), increasing the risk of infections.
✅
Explanation of Answer Choices:
D. Clozapine→Correct.Patients must be enrolled in theClozapine
❌
REMS program, and pharmacies must verifyANC and WBC resultsbefore dispensing.
A.
❌
Aripiprazole→ Incorrect.Aripiprazole (Abilify) does not require ANC monitoring.
B. Fluoxetine→
❌
Incorrect.Fluoxetine (Prozac) is an SSRI and does not require blood monitoring.
C. Amitriptyline→
Incorrect.Amitriptyline is a tricyclic antidepressant and does not require ANC monitoring.
Reference:
FDA Clozapine REMS Program
PTCB Exam: Medication Safety & Risk Evaluation
Question 12
Two stock bottles ofAtenololhave the following NDC numbers:
00781-1506-10and00781-1506-01. What is the difference between the bottles?
- A. Package size
- B. Dosage
- C. Manufacturer
- D. Strength
Answer:
A
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
TheNational Drug Code (NDC)is a10- or 11-digit numberidentifying a drug'smanufacturer, product,
and package size.
NDC Format:
XXXXX−YYYY−ZZ\text{XXXXX} - \text{YYYY} - \text{ZZ}XXXXX−YYYY−ZZ
First 5 digits= Manufacturer
Middle 4 digits= Drug, strength, and formulation
Last 2 digits=Package size
✅
A. Package size→Correct.Since the first two segments(00781-1506)are identical, the difference is
❌
in thelast segment, which representspackage size.
B. Dosage→ Incorrect. Thedosage (mg strength)
❌
remains the same.
C. Manufacturer→ Incorrect. Themanufacturer is the same (first 5 digits are
❌
identical).
D. Strength→ Incorrect. Thestrength is unchanged.
Reference:
FDA NDC Directory
PTCB Inventory Management Guidelines
Question 13
Desvenlafaxineis indicated to treat:
- A. Hypertension
- B. Major depressive disorder
- C. Benign prostatic hypertrophy
- D. Atrial fibrillation
Answer:
B
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
Desvenlafaxine (Pristiq)is anSNRI (Serotonin-Norepinephrine Reuptake Inhibitor)used to treatmajor
depressive disorder (MDD).
✅
Explanation of Answer Choices:
B. Major depressive disorder→Correct.Desvenlafaxine is FDA-
❌
❌
approved fordepression.
A. Hypertension→ Incorrect.Not an antihypertensive.
C. Benign
❌
prostatic hypertrophy→ Incorrect.Alpha-blockers like Tamsulosin treat BPH.
D. Atrial fibrillation→
Incorrect.Beta-blockers, not SNRIs, manage atrial fibrillation.
Reference:
FDA Drug Database: Desvenlafaxine
PTCB Exam: Pharmacology for Technicians
Question 14
According to theFDA,heparin strength per total volumeshould be the primary and prominent
expression on the manufacturer’s label, followed by the:
- A. Percentage weight per volume
- B. Volume per total strength
- C. Strength per mL enclosed in parentheses
- D. mL per dose enclosed in parentheses
Answer:
C
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
To preventheparin dosing errors, theFDA requires labeling to display:
Strength per total volume (e.g., 10,000 units/10 mL)
Strength per mL in parentheses (e.g., 1,000 units/mL)
✅
❌
Explanation of Answer Choices:
C. Strength per mL enclosed in parentheses→Correct.
A.
❌
Percentage weight per volume→ Incorrect.Heparin is not measured this way.
B. Volume per total
❌
strength→ Incorrect.Reverse order of required labeling.
D. mL per dose enclosed in parentheses→
Incorrect.Dosing varies by prescription.
Reference:
FDA Heparin Labeling Guidelines
Question 15
A prescriber orders1000 mgof a medication. If the stock bottle label stateseach tablet contains 0.1 g,
how many tablets should the patient receive?
- A. 1
- B. 10
- C. 100
- D. 1000
Answer:
B
Explanation:
Comprehensive and Detailed Step-by-Step Explanation:
A white background with black text Description automatically generated
✅
❌
Explanation of Answer Choices:
B. 10→Correct.
A. 1→ Incorrect.1 tablet contains 100 mg, not
❌
❌
1000 mg.
C. 100→ Incorrect.Overdose.
D. 1000→ Incorrect.Severe overdose.
Reference:
USP <795> Pharmaceutical Calculations
PTCB Dosage Conversions